



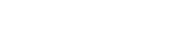


在氟烷基官能团中,二氟甲基性质独特,由于其与羟基及氨基在酸碱性和电性上相似,容易产生分子内以及分子间的氢键等次级相互作用,增强药物分子与酶之间的结合能力,因此药物化学家在设计药物分子结构时通常把二氟甲基当作是羟基及氨基的生物电子等排体。而如何在发展温和条件下向小分子引入二氟甲基是有机氟化学领域研究的难点。本课题组大胆地提出了一个双金属协同催化二氟甲基化的新策略。研究发现,钯催化的卤代芳烃的二氟甲基化反应的难点是把二氟甲基从TMSCF2H转移到金属钯中间体上,我们巧妙了设计了一个稳定的二氟甲基银试剂,该试剂在溶液里可以高效的实现二氟甲基的转金属,得到关键[(DPPF)Pd(Ar)(CF2H)]中间体,从这个关键中间体还原消除就得到二氟甲基取代的芳烃。在理解这个协同催化反应的基元反应的基础上,首次实现了溴代芳烃的二氟甲基化 (Nat. Commun. 2014, 5, 5405)。
Cooperative Dual Palladium/Silver Catalyst for Direct Difluoromethylation of Aryl Bromides and Iodides. The difluoromethyl group is often considered as biosiostere of hydro or thio group in drug design and considered as one of the privileged structural motifs that are important for fine-tuning the biological properties of drug molecules. Development of efficient method for the incorporation of difluoromethyl group has attracted increasing interests recently. The Shen group developed a cooperative dual palladium/silver catalyst for direct difluoromethylation of aryl bromides and iodides. The development such a system was based on initial preparation of the putative intermediates in the dual catalytic cycles, followed by studying the elemental steps to demonstrate the viability of the proposed cooperative catalytic cycle. The reaction is compatible with a variety of functional groups such as ester, amide, protected phenoxide, protected ketone, cyclopropyl, bromide and heteroaryl subunit such as pyrrole, benzothioazole, carbazole or pyridine (Nat. Commun. 2014, 5, 5405).

联系方式

友情链接